Cellular matrix BCT-HA
What is Cellular matrix BCT-HA?
Cellular Matrix (Class iii medical device) tubes enable the preparation of autologous platelet-rich plasma (Regen PRP) in combination with a non-cross-linked hyaluronic acid (HA) in a closed system. The resulting CELLULAR MATRIX Regen PRP-HA preparation is called CM-PRP-HA.
Regen PRP: The platelet concentrate prepared with Regen Lab technology provides an autologous reservoir of growth factors.
Hyaluronic acid is a key molecule for skin hydration and has the unique property of binding water 6-7.

As shown by Smith et al, fibrin polymerisation in the presence of HA creates a clot with large pores, which facilitates cell migration.9
Regen PRP® standardised performance
All Regen Lab products use the patented separating gel technology for the standardised production of cell therapy products with a reproducible cell profile:
BCT-HA® tubes properties | PRP volume per tube | Blood volume per tube | Platelet recovery | Granulocyte depletion | Red blood cell depletion |
|---|---|---|---|---|---|
Cell | ca. 3 ml | 6 ml | > 70 % | > 94.3 % | 99.5 % |
RegenBCT-HA Performance summary-v2, data on file
The volume of blood drawn with the BCT-HA tube is 4 ml, which allows the production of a final combination of 50% PRP - 50% HA with a volume of approximately 4 ml.
CELLULAR MATRIX is a unique, medical device approved, patented technology that enables the safe production of a cell-friendly PRP-HA network in a closed system where platelets and plasma components are preserved.
Preparation in 3 steps in less than 10 minutes:
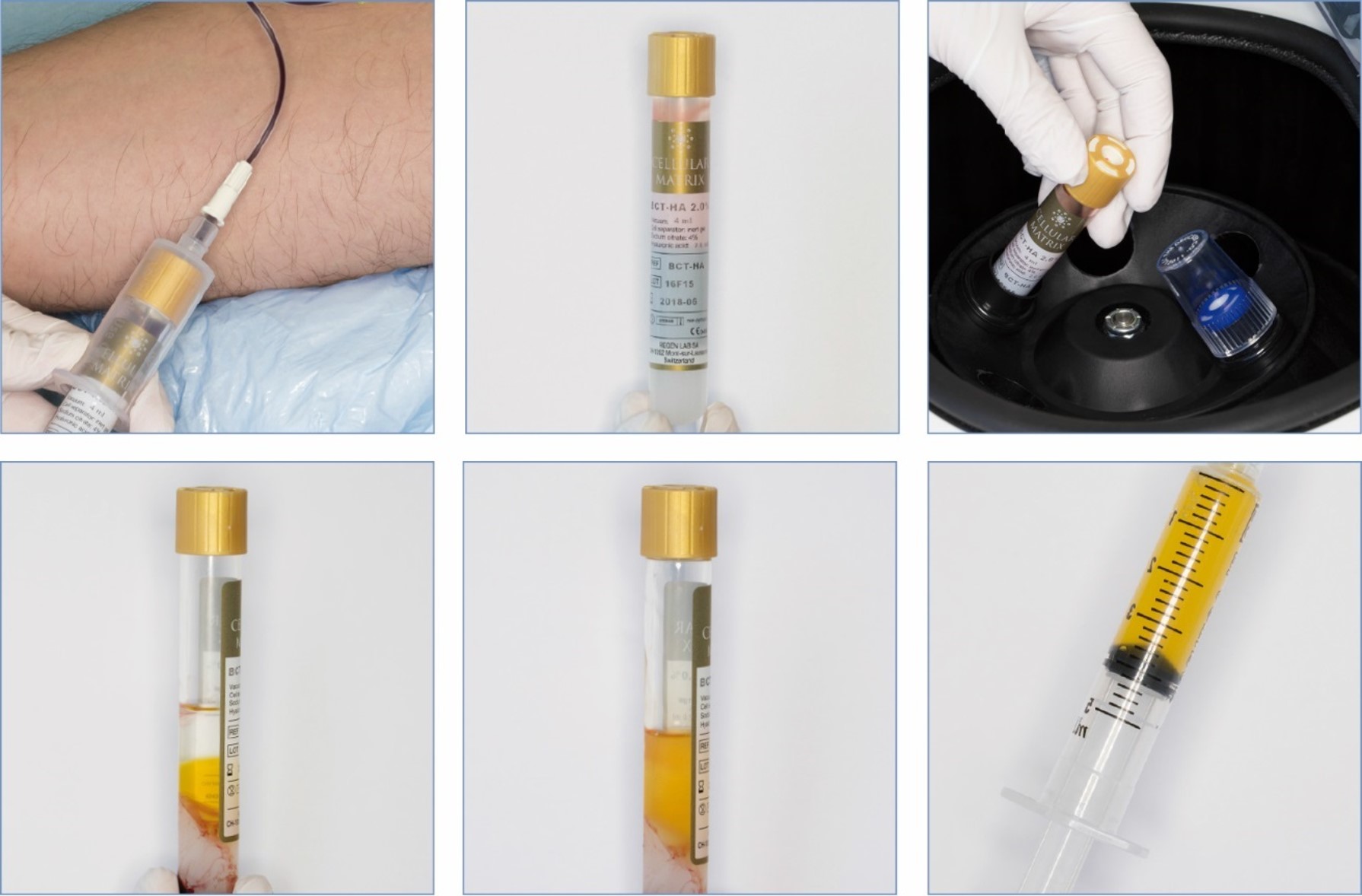
Please always read the operating instructions before use:
Intended use of the product:
Product for the preparation of intra-articular injections into the knee for the treatment of articular pain and improvement of mobility.
Class III CE certified medical devices Regen Lab SA is an ISO13485 :2016 and MDSAP certified medical device manufacturer.
CE2797
CONTRAINDICATIONS:
Absolute contraindications: The product must not be administered to patients who have been diagnosed with hypersensitivity to any of the components, or who have a serious disease such as cancer or inflammation of the joint or the area to be treated. Do not use in patients with hereditary or acquired blood disorders/coagulation disorders such as platelet dysfunction, critical thrombocytopenia, impaired coagulation or in patients with uncontrolled serious metabolic disorders or systemic diseases. The use of PRP/HA preparation is not recommended in patients with partial knee prosthesis. Relative contraindications: Administration of the PRP/HA preparation to patients suffering from inflammatory joint disease or autoimmune disease such as rheumatoid arthritis or ankylosing spondylitis is not recommended. Administration to children, pregnant or breastfeeding women is not recommended. If medications or supplements that affect platelet function are taken within 3 days, this may affect the effectiveness of the treatment.
Use of the PRP/HA preparation is not recommended in patients with local skin disease at the injection site.
POSSIBLE SIDE EFFECTS:
Possible side effects during blood collection: blood collection may result in damage to blood vessels, haematoma, superficial phlebitis, delayed wound healing and/or infection, temporary or permanent nerve damage, which in turn may result in pain or numbness and early or late infection.
Possible side effects with intra-articular: Injection With intra-articular injection, secondary inflammatory reactions localised to the injection site may occur. This can lead to temporary pain, a feeling of heat, redness and swelling at the joint treated with the PRP/HA preparation. Ice packs a few minutes after the injection or treatment with local anaesthetics the day after the injection can alleviate this discomfort. The use of non-steroidal anti-inflammatory drugs (NSAIDs) must be avoided. Hypersensitivity, rarely anaphylaxis, has also been reported in a few cases. Pronounced inflammatory reactions have also been reported after the use of HA. The injection may lead to infections if the general precautions for injection and asepsis are not observed.
(Contraindications and side effects according to IFU)



