Cellular Matrix A-CP-HA
What is Cellular Matrix?
Cellular Matrix tubes enable the preparation of autologous platelet-rich plasma (Regen PRP®) in combination with a non-cross-linked hyaluronic acid (HA) in a closed system.
Regen PRP: Platelet concentrate prepared with Regen Lab technology provides an autologous reservoir of growth factors.
Hyaluronic acid enhances the activity of several molecules found in platelet-rich plasma to provide additional benefits to patients with OA.1
Both PRP and HA have been widely used to improve joint lubrication, relieve inflammation and influence the catabolic microenvironment of the joint, with the aim of not only reducing clinical symptoms but also slowing the progression of OA.6
When PRP and HA are used in combination, these effects are enhanced and prolonged. HA forms a bioactive scaffold in which platelets gradually release their growth factors. Rain PRP has no negative effect on the mechanical, elastic or viscous properties of HA.1
Cellular Matrix is a unique, medical device-approved, patented technology that enables the safe preparation of a cell-friendly PRP-HA network in a closed system where platelets and plasma components are preserved.
PLATELET-RICH PLASMA
HYALURONIC ACID
CELLULAR MATRIX TECHNOLOGY
Regen PRP® standardised performance
All Regen Lab products use the patented separating gel technology for standardised production of cell therapy products with reproducible cell profile:
ACP-HA® | PRP Vol. per tube | Blood volume per tube | Platelet recovery | Red blood cell depletion | Granulocyte depletion |
|---|---|---|---|---|---|
Cell | approx. 3 ml PRP combined with 2 ml HA | 6 ml | > 70 % | 99.5 % | 94.3 % |
A-CP-HA performance summary-v7ml, data on file
The volume of blood collected with the A-CP-HA tube is 6 ml, which allows the preparation of a final combination of 60% PRP - 40% HA with a volume of about 5 ml.
Preparation in 3 steps in less than 10 minutes:
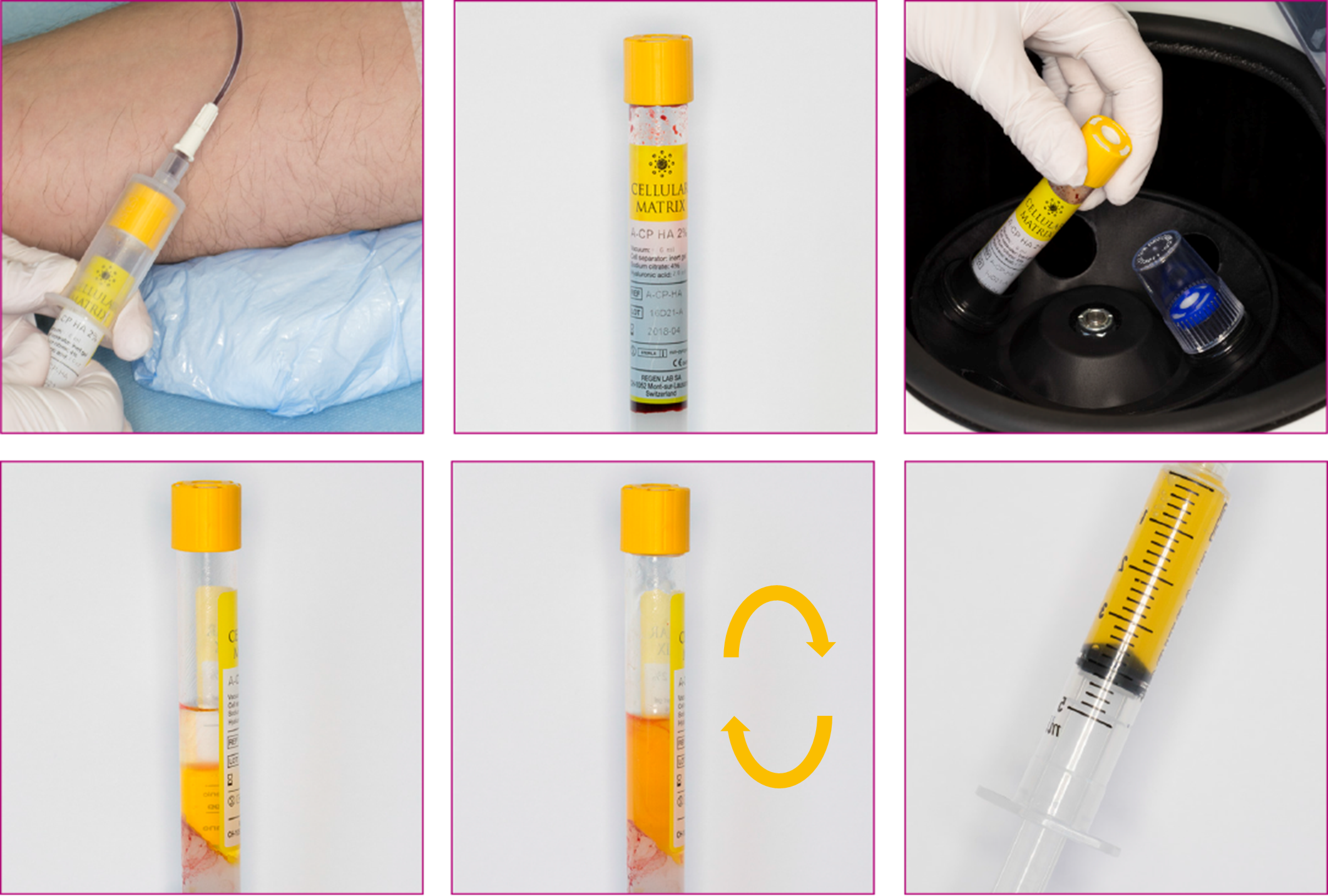
Please always read the operating instructions before use:
Intended use of the product:
Preparation of autologous platelet-rich plasma and other plasma-based products.
Class IIb CE certified medical devices
Regen Lab SA is a medical device manufacturer certified to ISO13485 :2016 and MDSAP.
CE2797
Warnings and precautions:
Sterility must be maintained throughout the phlebotomy procedure. Take adequate precautions to avoid contact with the patient's blood or cross-contamination. Take adequate precautions to protect against needles or broken glass tubes. Do not use any of the sterile components of the kit if the packaging has been opened or damaged. Do not use any component of this kit that is damaged or broken. Do not use the tube if it has lost vacuum. Do not use the sodium citrate or other components of the tube alone. Store between 5ºC and 30ºC; bring the kit to room temperature before use. Do not use after the expiry date. Disposable; do not reuse any part of the kit. Reuse may cause infection or other
illness/injury.
Transfer needles must only be used for fluid transfer or preparation of the injection, but not for the injection itself. Preparation of platelet-rich plasma (PRP) must be performed by or under the supervision of a physician trained in the device and procedures. PRP preparation must only be used by a doctor trained in the procedures. The patient must be informed of general risks and possible adverse effects. The platelet-rich plasma (PRP) must be prepared with fresh blood and used within four hours (product, use as soon as possible and do not store). Dispose of the tubes and other components after use according to the disposal guidelines for potentially contaminated blood products.
Use a centrifuge with swing-out or fixed-angle rotor (e.g. RegenPRP Centri from Regen Lab). Follow the manufacturer's instructions when using the centrifuge. The tubes should be centrifuged at 1500 RZB as described in the instructions for use. High centrifugal forces (greater than 2200 RZB) may cause the tubes to break, come into contact with the blood or possibly cause injury. A lower centrifugal force (less than 1500 RZB) may result in insufficient separation of the blood and contamination of the PRP with red blood cells. The centrifuge inserts should specifically match the size of the tubes. The use of inserts that are too small or too large may cause the tubes to break. Care should be taken to ensure that the tubes are properly seated in the holder in the centrifuge. The tubes must be balanced in the rotor to avoid possible breakage of the glass.
Possible undesirable side effects:
Injury to blood vessels, haematoma, delayed wound healing and/or infection. Temporary or permanent damage to nerves which may result in pain or numbness. Early or late surgical infection.



